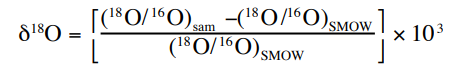Fall 2019
Mentor: Dr. Julie Bryce
Applications of δ18-O to Mid-Latitude Alpine Glaciers for Paleoclimatic Research: Examples from the Wind River Range, WY
Introduction
Stable isotopes in water are affected by meteorological regimes and atmospheric conditions that can provide a unique identifier of their origin. Analyzing the composition, concentration, and stratigraphic distribution of δ18O in ice cores is an effective method for paleoclimate reconstructions that has been demonstrated in numerous sites, most famously in the ice sheets of Antarctica and Greenland, but in small alpine glaciers as well (e.g., the high Andes). δ18O in well-preserved ice can be used as a proxy for temperature, and with ancillary geochemical data for piecing together a record of hydroclimate change. Prior to the research that was conducted in the Wind River Range (WY), mid-latitude glaciers were thought to have minimal potential to reveal reliable information on paleoclimate. The uncertainties are generally attributed to meltwater percolation effects and the broad range of oxygen isotope abundances as a response to higher seasonal variability in these regions. In this paper, I discuss: (a) the basic processes and controls of oxygen isotope speciation, (b) paleoclimate applications of δ18O to polar ice sheets, (c) the motivation behind applying δ18O geochemistry to ice cores from the Wind River Range, (d) the calibration and geochronology methods specifically used in these mid-latitude glaciers, and (e) key evidence of the Little Ice Age from Wind River ice cores, including a the development of a refined age-model created from δ18O and electrical conductivity measurements, and a transfer-function used to establish mean annual temperature changes using δ18O.
Fractionation & Oxygen Isotopes
Figure 1: Equation for delta18-O. (Clark & Fritz, 1997)
To investigate the application and use of δ18O glacial ice, we must first understand the basic process that allows different isotopes of oxygen to occur from fractionation and how these isotopes are measured. The species of oxygen isotopes is a function of temperature and is strongly influenced by certain meteorological and hydrological processes.
Isotopic Fractionation
Isotopes of the same element have different masses, resulting from a difference in the number of neutrons, which helps to account for varied reaction rates that can facilitate fractionation or partitioning of a certain species in a reservoir. Physiochemical fractionation is characterized by faster reaction rates in lighter nuclei than heavier isotopes due to their smaller bond strength. Heavier isotopic species are more commonly partitioned into a more condensed phase, such as the solid phase in mineral-solution reactions or the aqueous phase in vapor-liquid interactions (Clark & Fritz, 1997).
Oxygen Isotopes
Stable isotopes are measured as ratio of the two most abundant isotopes for a given element. Measured ratios are compared to a reference standard to produce a difference expressed as parts per thousand (per mil, ‰). For oxygen isotopes, 18O has a terrestrial abundance of 0.204% and 16O has a terrestrial abundance of 99.796%. Thus, the ratio is about 0.00204. Ratios determined in a given sample are referenced to SMOW, the Standard Mean Ocean Water. Oxygen isotope fractionation is mass-dependent, which means that it is proportional to the mass gradient or difference. Lighter molecules have greater translational velocities, which allow them to emerge from a liquid surface more easily and evaporate more readily than the heavier molecules. Hydrogen fractionations are related to oxygen isotopes in precipitation, however hydrogen is greater because the mass difference is greater. Despite the fact that only 10% of evaporated moisture from the oceans reaches the continents, a variety of partitioning pathways can occur by process such as rainfall, re-evaporation, and snow and ice accumulation. Oxygen and hydrogen isotope behavior can usually be be very confidently predicted through the meteoric water line (MWL) (Clark & Fritz, 1997). The MWL describes the astonishing correlation of δ18O and δ2H in freshwater reservoirs on a global scale. For example, isotopically depleted waters are commonly found in high latitude cold regions while those that are enriched by evaporation are located in warmer regions in the tropics. Typical values of δ18O for Greenland fall in the range of -30 to -35‰, while ice in Antarctica can exhibit δ18O values as low as -50‰ (Clark & Fritz, 1997).
Meteorological Controls
Temperature regulated mechanisms have potentially large effects on the stable isotopic composition of precipitation. These mechanisms include the Latitudinal Effect, Continentality Effect, and Altitude Effect (Clark & Fritz, 1997).
Latitudinal Effect
In general, precipitation at higher latitudes produce more negative δ18O. δ18O Temperature gradients are -0.6‰ for δ18O per degree of latitude for North American continental stations (Clark & Fritz, 1997). Water can exist in several phases, including solid (ice), liquid (water), and gas (vapor), and temperature and pressure changes can alter the phases. Strong seasonal variability and the range of temperatures related to latitude (especially in places like North America) offer a significant control on the isotopic evolution of vapor and precipitation, and therefore broad differences in δ18O should be expected between areas like Florida versus New Hampshire .
Continental Effect
Isotopic composition of water vapor evolves as it moves from its ocean source inland. This is caused by topographic effects and temperature extremes that are common for continental interiors that aren’t moderated by marine influences (Clark & Fritz, 1997). As a result, continents can strong seasonal variations in temperature. This effect is described by continentality (k), where the average annual range in temperature is related to the latitudinal angle. This relationship is given by the equation: K = (1.7ΔT / sin(ϕ+10)) – 14 (Clark & Fritz, 1997).
Altitude Effect
Temperature and altitude are normally inversely related: as elevation increases, temperature declines, and this can be seen in the vegetation and snowlines on many mountains. Depletion of 18O varies between -0.15‰ and -0.5‰ per 100 m rise in altitude (Clark & Fritz, 1997). At high altitude, sublimation – the process by which snow is converted to vapor – and other modifying processes can also influence the δ18O (Naftz et al., 2002). The orographic precipitation influence on oxygen isotope fractionation is evident where mountains force air masses up to a cool altitude that can facilitate condensation and precipitation. Thus, precipitation on the leeward side of a topographic high point will be isotopically lighter than on the windward side.
Process Related Variability: Rain
The process of rain cloud formation and ultimate releasing of these condensed particles has a particular influence on the variability of oxygen isotopes.
Raleigh Distillation
Raleigh distillation is a process through which isotopically enriched rain is formed and falls as wet deposition from a diminishing vapor mass and this residual vapor (f) is progressively depleted as a result. R = R0 fα-1 (Clark & Fritz, 1997) This process leads to changes in the isotopic composition of rainfall across long distance storm tracks, in spite of a common starting point or moisture source.
Rain Formation
The isotopic evolution of precipitation during rainout is determined by temperature. Since rain is formed when a vapor mass is cooled adiabatically by rising to a region in the atmosphere with lower pressure, it eventually reaches a temperature at which there is 100% humidity (i.e., the dew point), allowing it to condense and fall as rain or snow (Clark & Fritz, 1997). Thus, the temperature within the cloud helps to determine what species of O is partitioned and condensed and not the surface or local temperature. However, given the difficulty in monitoring internal cloud temperatures, relationships of 18O variability are deduced using local mean temperature data.
Rainout
Research has shown that δ18O of ice may be controlled by δ18O of precipitation, which is dominated by moisture supply and storm trajectories. Rainout is involved in the process where a trajectory (wind patterns, weather systems, etc.) directs an air mass away from its evaporative source and over a continent to higher latitudes and potentially altitudes. The air mass experiences several episodes of cooling and loss of water vapor by precipitation along this path, successively distilling the heavy isotopes from the vapor and become progressively depleted in 18O and 2H (Clark & Fritz, 1997).
Significance
Analyzing the composition, concentration, and stratigraphic distribution of δ18O in ice cores is an effective method for paleoclimate reconstructions. Isotope studies made on ice cores are widely referenced in Quaternary studies, and similar to marine isotope records made from foraminifera, are considered to be important tracers of global environmental changes (Ruddiman, 2001). In a typical ice core, isotopically enriched summer layers alternate with more depleted winter horizons (Clark & Fritz, 1997). Using this relationship, δ18O in wellpreserved ice can be used as a proxy for temperature, which is one of the most important variables for understanding climate history, especially in the Quaternary. These δ18O are sometimes combined with other ice measurements that be used to learn about atmospheric composition, volcanic processes, and more.
Glacial Studies
The application of oxygen isotopes as a proxy for paleoclimate was led by the pioneering work of scientist Willi Dansgaard, one of the namesakes for Dansgaard-Oeschger cycles (Ruddiman, 2001). Following the release of several comprehensive publication on the subject of oxygen and hydrogen isotopes in ice starting in the 1950’s, a progressively improved understanding of these isotopes was bestowed upon the scientific community, which led to greater application and improved interpretations. The Greenland Ice-Core Project (GRIP) was established in the early 1990’s and since that time, foundational papers have been produced detailing the variability of δ18O in precipitation and ice records in response to local environmental conditions and global atmospheric interactions. Two of these early papers together have been cited more 3000 times (Grootes et al., 1993, Johnsen et al., 2001). One prominent discovery was rapid climatic changes in the Pleistocene, which are the abrupt changes in δ18O (several per mille each) and temperature (signifying a rapid warming and slower cooling) that make up the Dansgaard-Oeschger cycles. The cycles occurred approximately every 1500 years and their causes have been debated (Ruddiman, 2001). One possibility is ice sheet instability and breakup, while another revolves around changes in greenhouse gases in the atmosphere. Another key finding from Greenland was the occurrence of big changes in deglacial and Holocene climate, such as the Younger Dryas and the 8,200 year event. In particular, the Younger Dryas appears as a big and abrupt shift to lower δ18O in Greenland Ice (Grootes et al., 1993). This led scientists to conclude that cold periods could affect the earth even during a major phase of deglaciation. The relationship of oxygen isotope fractionation with respect to mean annual temperature has been recognized by correlations observed in Greenland ice cores over long time scales in the Pleistocene and has even been linked to specific snow storm events and their temperatures more recently. The Antarctic record in the southern hemisphere also has been explored for temperature data using δ18O, and these cores go back farther in time than the Greenland data. These distinct and relatively clear patterns in temperature and oxygen isotopes have only been observed in the polar regions; mid-latitude sites in the USA provide limited insights into these anomalous systems, which will be discussed more in relation to the Wind River Range (Naftz et al., 2002).
Despite the surge of oxygen isotope studies on the polar ice sheets, minimal efforts have been made to investigate the variability of δ18O in mid-latitude alpine glaciated regions. This is why I will discuss only a handful of papers pertaining to the Wind River Range that were published mostly in the late 1990’s.
Figure 2: EOS Article. Vol. 73, No. 3, January 21, 1992.
Applications in Mid-Latitude Glaciers in the Wind River Range
For sites in the mid-latitudes, δ18O of ice can be more difficult to interpret, and may be more closely associated with the average annual air temperature than precipitation delivered by storm paths (Naftz et al., 2002) since mid-latitudes have much higher seasonal temperature variability than polar regions. Prior to the research that was conducted in the Wind River Range, mid-latitude glaciated regions were considered to have minimal potential to reveal reliable information on paleoclimate. However, the data produced from USGS scientists in these inaugural studies document some similar patterns with respect to other ice cores obtained from higher latitudes and elsewhere.
January, 1992, a scientific newspaper (EOS) published a short column about a USGS drilling project team that had successfully collected a 160 m long ice core from Fremont Glacier in 1991. This drilling project had been active in climate research studies on temperate glaciers in the Wind River Range since 1988, mostly in an effort to determine the chemical quality of the atmospheric deposition as a consequence of upwind sources of pollution and particulates. The chemical composition of glacial ice can provide a useful record of constituents previously suspended in the atmosphere that are deposited in the wet (precipitation of rain and snow) and dry (dust particle fallout) forms. In the 1980’s, the national atmospheric deposition program (NADP) was established by the federal government to monitor wet deposition of atmospheric pollution. This record is relatively short and the what is found in downwind glacial ice can extend the record back in time beyond the time of the NADP database. The ice coring project was in partnership with several official organizations including the Shoshone and Arapaho Indian tribes, PICO, BLM, and the WY Water Development Commission to legitimize the importance of atmospheric non-point source pollution.
Wind River Geographic Setting
The Wind River Range is located in central Wyoming and is the highest and longest mountain system in the state, containing the highest peak (Gannett Peak at 13,804 ft) and extending 80 mi with an approximate N-S strike within the greater Rocky Mountains. The greatest concentration of glaciers for all of the Rockies, including 7 of the 10 largest glaciers in the contiguous USA, are found in the Wind Rivers. The location and geography of the Wind Rivers is important to Rockies hydrology. The continental divide spans the crest of the range and joins a third divide – the Green River headwaters - on the north end that creates a triple divide for the Columbia River watershed in the west, Missouri River in the east, and upstream Green River portion of the greater Colorado River basin. The glaciers studied in Wind Rivers include Knife Point, Gannett, and Upper Fremont Glacier. A study done in the nearby Absaroka Mountains investigated Galena Creek Glacier to compare to Upper Fremont. All four glaciers are situated on the eastern side of the Continental Divide.
Early Studies: Meltwater Percolation and Chronological Considerations
Meltwater production and percolation within ice bodies can create issues for paleoclimate studies in the mid-latitudes (Cecil et al., 1998). These effects may dampen seasonal δ18O signals and cause a shift to less negative δ18O values (Naftz et al., 1996). This issue was a big concern for the researchers trying to extract climate signals from the Upper Fremont Glacier ice core, even though care was taken to collect ice from the accumulation zone at high elevation, where meltwater should be minimized.
Figure 3: Concentrations of tritium in ice samples compared to depth from the Knife Point Glacier, representing the time between 1987-1980 and a 2.5m maximum depth. (Fig. 2, Naftz et al., 1991)
35-S and Tritium at Galena Creek Glacier
To distinguish between recent and older sections of ice and to test for meltwater contributions, an analysis of the abundance of 35S, a radioactive nuclide produced by cosmogenic rays, was used because of its very short halflife of 87 days (Cecil et al., 1998). Ice and meltwater represented in the core should have no 35S prior to 1940 and may contain very small amounts of 35S after 1980 (Cecil et al., 1998). 35S is quickly oxidized to form sulfate and it gets easily incorporated into glacial ice upon deposition. The meltwater composition of 35S in Galena Creek expressed a low value of about half of what would be observed in modern precipitation (5.4mBeq/L). Considering the short half-life, this suggested that recent melting had occurred from accumulation that was deposited within the last two years. In other words, the signal represents meltwater from recent precipitation, because there was no apparent contribution from older ice. This finding was important because it implied to the authors that Galena Creek glacier and Upper Fremont glacier were experiencing slow rates of ablation. Galena Creek ice was also analyzed for 3H, in order to distinguish between older and younger ice and to see if sources of meltwater percolate into and disrupts the ice stratigraphy. The values found in the Galena Creek Glacier were similar to modern precipitation, suggesting old meltwater contributions are insignificant in the ice record.
Atmospheric Deposition of Major Ions at Knife Point Glacier
To identify layers of ice that had not been contaminated by meltwater, the abundance of major ions left by atmospheric deposition was investigated in an ice core from Knife Point Glacier (Naftz et al., 1991). To minimize this risk of meltwater percolation interference, ice was again collected at elevations greater than 3500 m asl. The purpose of this study was to examine annual layer preservation, to see if Wind River glaciers could be accurately dated, and to determine if the chemical composition of the ice is related to wet deposition as opposed to atmospheric fallout (dust or dry deposition).
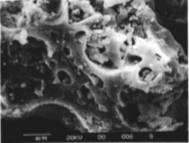
Figure 4: Photomicrograph of Mount St. Helen’s ash collected near Kellogg, Idaho on May 21, 1980 representing the vesicular structure of the ash grains. (Fig. 3, Naftz et al., 1991)
In order to use ice cores to reconstruct different aspects of climate or pollution history, a chronology of ice accumulation is necessary. However, several studies showed that the mid latitude glaciers did not preserve extensive laminations like those seen in high-latitude glaciers (Naftz et al., 1996). Knife Point contained a few prominent laminations, and this study focused on a short interval of time using a trench surface dug into the ice. Unlike some of the other Wind River glaciers, Knife Point showed annual layering marked by thin laminations of gray colored dust. A useful chronological marker was Mount St. Helens’ last eruption (1980), which dispersed copious volumes of ash into the atmosphere that is recognized in sediment or ice archives by ash with silica enrichment and the presence of vesicles. Geochemical analysis of these dust in Knife Point revealed a particular layer that was compositionally similar to the ash deposited by this Mount St. Helens eruption (Naftz et al., 1991). Another useful chronological marker was the appearance of trichlorofluoromethane (CCl3F). CCl3F is produced by industrial reactions and is not otherwise found in the naturally occurring environment. Beginning in 1931, humans began producing this compound. Where CCl3F concentrations were detectable in the ice, it indicated an age younger than 1931 (Naftz et al., 1991). The last time marker that was evaluated was tritium (3H). Nuclear weapons testing conducted in the 1950’s and 1960’s released large amounts of neutrons in the upper atmosphere that were subsequently bonded with other atmospheric isotopes and exposed to cosmogenic radiation via spallation to produce acute concentrations of unstable and radioactive isotopes, including 3H (as well as 36Cl). Following formation, these molecules then attached themselves to aerosols in the atmosphere resulting in ‘fallout’, or deposition as precipitation or dust, thus allowing the rapid elimination of these particles from the atmosphere. Geochemical records in ice cores document the distribution and abrupt reduction of radioactive nuclides in the atmosphere by deposition since nuclear weapons testing. The production and abrupt atmospheric concentrations of 3H and 36Cl can be used by as precise geochronometers since the concentrations on either side of the time of nuclear testing – pre and post 1950’s-1960’s – should be very low (Cecil et al., 1998). From the Knife Point glacier, the 3H record shows an increasing abundance with depth between known ages of 1980 to 1987 (Naftz et al., 1991).
From the major ion analysis, abundances of Ca, Mg, Na, Cl, and SO4 were measured and compared to wet deposition concentrations recorded by the NADP in Pinedale, WY. Of these elements, only Ca, Cl, and SO4 were positively correlated with NADP, although the sulfate correlation was still relatively poor. The authors argued that because the correlation exists, then meltwater must not be contaminating Knife Point glacier, and that long ice cores could provide a great deal of contaminant and climate history information.
Upper Fremont Glacier - Initial Chronology
Upper Fremont was most important for the USGS ice core studies, as it was the longest and oldest glacial record from the Wind Rivers. Several samples from the 160 m core were dated using radiocarbon. A majority of the dates produced from these samples yielded large uncertainties and were consequently discredited – except for one. A grasshopper leg found at 152 m was determined to be 1716-1820 AD (221 +/- 95 bp). This age, coupled with the knowledge of the exact species of the grasshopper, provided the ice core with at least one conclusive age to evaluate modern accumulation rates and climate history (Naftz et al., 1996). A number of other methods were used to build up the chronology of the Upper Fremont core. For example, the concentration of 36Cl was measured, which as discussed above, is related to nuclear weapons testing. The 36Cl peak was found at the 32 m depth (Cecil and Vogt, 1997). Using tritium, Cecil et al. found no evidence of meltwater contamination at depth in the Upper Fremont ice. 3H levels from the Upper Fremont glacier ice core displayed a marked peak at a depth of 29 m, exceeding 300TU. This boundary layer corresponds to when above ground nuclear weapons testing was most frequent in the early 1960’s (Naftz et al., 1996).
Evidence for the Little Ice Age Anomaly in δ18-O and nitrates
Figure 5: Relation of the d18O values with depth in the 160m Upper Fremont Glacier. Shaded section (100-150m depth) represents section of high-amplitude oscillations identified in the Upper Fremon Glacier ice-core record. (Fig. 5, Naftz et al., 1996)
δ18O measurements taken from the ice core extracted from Upper Fremont glacier revealed signatures consistent with the Little Ice Age (LIA) (Naftz et al., 1996). Within the Upper Fremont ice core, the average δ18O over the entire 160 m core was -18.9‰. Significant isotopic variations in the core are mostly in the lower part of the core, below 101.8 m depth, where the average δ18O value is -19.85‰. Overall, the core exhibits more negative δ18O and high amplitude oscillations of δ18O during the LIA, compared to as less variable δ18O after the LIA (Naftz et al., 1996). Radiocarbon dates from deep ice sections indicate that the more negative δ18O ice formed in the mid-1700’s to mid-1800’s. These ages correlate with temperature reconstructions done for Europe which correspond to the end of the LIA. Similar studies done on tree rings in the western US also confirm this was a cold period in North America (Naftz et al., 1996).
Greater δ18O variability shown in the deeper ice from the mid 1700-1800’s might represent conditions of either more summer precipitation (when δ18O are more positive) or more winter precipitation (when δ18O are more negative). Colder LIA summers could have also preserved the δ18O signal better by limiting seasonal melting (Naftz et al., 1996). The LIA and post-LIA oxygen isotope compositions in this core are further substantiated by comparing the δ18O composition to the famous Quelccaya ice core from the Peruvian Andes. Both cores exhibit more negative δ18O and high amplitude oscillations of δ18O during the LIA, as well as less variable compositions after the LIA (Naftz et al., 1996). Nitrate concentrations in the Upper Fremont Glacier were very low during the LIA, and low nitrate concentrations were observed in Greenland ice during glacial intervals. A possible explanation can be attributed to less nitrogen-fixing bacteria in the snow during these colder episodes. More positive δ18O and nitrate concentrations in the bottom 10 m of the core may be an effect of a basal meltwater layer and freezing/refreezing at the bedrock contact (Naftz et al., 1996).
Development of a Refined Age-Model using Electrical Conductivity
Figure 6: Nitrate concentration profile from UF core with depth (0-160m). (Fig. 3, Naftz et al., 1996)
Electrical conductivity (ECM), sulfate and chloride concentrations were measured for Upper Fremont ice core by Schuster et al. (2000), and the results were compared to chemical age dates and the 18O profile provided by the previous research to correlate acidic atmospheric fallout from volcanic events with core depth, in order to refine the chronological history of the Upper Fremont Glacier. ECM is a direct approach to measuring the acidity of ice. Particulate products of volcanic eruptions and anthropogenic industrial activities, such as sulfate and chloride, are acidic and get released into the atmosphere, eventually falling out by a wet or dry depositional process, accumulating in ice and increasing the electrical conductivity. Core samples from depths greater than 15m (below the firn layer, where ice was compact) consistently exhibited significant CL- and SO42- peaks where there is also a significantly high ECM signal. ECM can help detect events where evidence has been altered. For example, EDA and SEM measurements identified the Mount St. Helens ash layer in Knife Point glacier, about 4mi SE of Upper Fremont. This ash layer was not visible in the Upper Fremont ice core, suggesting meltwater may have removed ash particulates from the surface, only leaving behind the residual acidity that was detected by ECM. Schuster et al. (2000) outlined the elution sequence of major chemical components removed through meltwater percolation effects in glaciers (SO42- > NO3-, NH4+, K+, Ca2+, Mg2+, H3O+, Na+, Cl-). This represents the order in which major ions are removed from ice by meltwater during warm summer months, a process that mid-latitude glaciers are especially vulnerable to and has been observed in Wind River glaciers (Naftz et al., 1993). Thus, any preserved chemical signal in the ice may reflect a diminished fraction of what was initially deposited. The lower 10 m of core reflects enhanced chemical variability, similar to those observed in the 18O. Between 150-155m depth, Cl- concentrations exceed the highest Cl- concentration recorded in the rest of the core by more than two times. Elevated Cl- and no considerable ECM signal at this basal depth suggests the Cl- more likely originates from chemical interactions at the ice-bedrock interface and not atmospheric origins.
Schuster et al. (2000) expressed dissatisfaction with the rudimentary age model provided in Naftz et al., even noting the grasshopper leg radiocarbon age was only moderately credible because of a 95 yr error bar and failing to consider post-depositional movement within the core. Schuster et al. (2000) suggested that the chemical dates determined from 3H, 36Cl, and radiocarbon outlined in Naftz et al. (1993) provide a “preliminary” age-depth profile. This initial model predicted that deposition for the layer at 88 m depth occurred in 1881. This depth is coincident with the highest ECM signal, representing one of the largest volcanic events in history from the eruption of Krakatau in Indonesia in 1883. Atmospheric dust from this event was observed in North America just a few months after the eruption. Thus, the layer at 88m depth in Upper Fremont was deposited in 1883, instead of 1881. The second largest ECM peak in the core occurs at 123 m. The initial age model proposes an 1805 AD time of deposition. Another massive volcanic eruption occurred in Indonesia at Tambora in 1815, releasing ash into the atmosphere that was observable in North American and England skies for nearly 6 months almost immediately after the eruption. Thus, the layer at 123m depth in Upper Fremont was deposited in 1815, instead of 1805. The refined agemodel is based on these two prominent dates and ECM signals correlating to the 27 most recent and significant volcanic (Figure 8, Figure 9). This refined profile is in good agreement with radiocarbon age of the grasshopper, yielding 1736 A.D. deposition at 152m depth.
Figure 7: Profile of a 999-point running average of ECM data with depth (0-160m) from the UF glacier showing a decrease in variance at 108m. (Fig. 4, Schuster et al., 2000)
δ18O variance increases abruptly in the lower half of the Upper Fremont ice core beyond 108m depth, previously determined to be a response to LIA climate. However, due to the coarse (20 cm) sampling resolution for δ18O, the end of the LIA was indeterminate. Comparing ECM and 18O profiles, ECM values show a coincident abrupt shift to decreased variance at this boundary. The refined age model yields an age of 1845 A.D. at this depth, representing the LIA termination and abrupt shift to warmer temperatures. Confidence and prediction limits suggest the time taken to complete the LIA climatic shift to present-day climate was about 10 years – meaning LIA termination in mid-latitude alpine regions of North America happened over a relatively short time-scale.
Figure 8: Plot of reported volcanic events and isotopic age dates used to generate a polynomial fit for an age-depth profile of the UF ice core. (Fig. 6, Schuster et al., 2000)
Using δ18-O to Reconstruct Temperature Changes with Transfer Functions (Naftz et al., 2002)
There have been a few studies that attempt to extract additional information from the Upper Fremont δ18O record, in spite of its shortcomings. In this study of Naftz et al. (2002), a transfer function, a statistical relationship relating temperature of snow storms to the δ18O in the deposited snow/ice, was developed and ultimately applied to Upper Fremont using the revised Schuster et al. (2000) age model. Transfer functions are unforgiving and must be developed for a specific site. This study was done by setting up a weather station with accurate sensors that recorded hourly weather metrics over several years, including temperature and snow accumulation depth for every storm. Transfer functions were made for four time intervals, each spanning a year. In the years where temperature and δ18O are related, the slope of the linear relationship changes. These slope changes may be explained by convective cloud droplets and reevaporation during late spring or summer snow events. Redeposition of snow and removal of snow by wind can influence the snow depth measurements and isotopic composition at the surface of the glacier. Wind sensors on the glacier indicate dense early season snows are less susceptible to removal by wind, while colder temperatures after October generate less dense snow that can be transported by wind more easily. All of these processes are relevant for making the transfer functions effective as describing temperature changes in the distant past.
Two ice cores from two different sites on the Upper Fremont glacier collected in 1991 and 1998 both show a significant isotopic enrichment of δ18O in the upper 40m, indicative of a warming trend in annual temperatures from the early 1950’s to early 1990’s (Figure 10). To reconstruct the annual temperature from ice core data collected at UFG in 1998, a transfer function from site-specific air temperature and δ18O values in snow deposited on the UFG was applied. The plot of these annual temperature changes with respect to δ18O values from the 1998 core suggests 3.5°C of warming from the mid-1960’s to early 1990’s (Figure 11). The same transfer function was applied to depths of 102150m in the 1991 core and yielded a positive 5°C change in temperature between the end of the LIA (mid1800’s) to early 1900’s. These reconstructions are substantially higher than the global average of 1°C of warming for the 20th century. However, similar changes in mean annual temperature over this time period has been calculated from select high-altitude, high-latitude polar regions, suggesting the mid-latitude alpine glaciers are extremely vulnerable to warming.
Conclusions
1. Oxygen isotopes can be used learn about climate and hydrology because of the manner in which 18O and 16O move through the atmosphere and hydrosphere. Major controls on δ18O in precipitation include latitude, altitude, moisture source, continentality, and of course temperature. The different number of neutrons between the isotopes allows for easy measurements using basic mass spectrometry.
2. Studies of Greenland ice δ18O has led to major discoveries in Quaternary paleoclimate, including abrupt and short-lived changes in temperature, such as D-O cycles and the Younger Dryas. Ice cores from Greenland are shorter than those from Antarctica but give important information about the climate of the northern pole region.
Figure 9: The d18O values measured in the upper 40m of two ice cores from Upper Fremont Glacier. A) Ice core collected in 1991. B) Ice core collected in 1998. (Fig. 10, Naftz et al., 2002)
3. Mid-latitude glaciers are not well studied using ice cores and δ18O. This is most likely because of the climate of these regions, which have warm summers that can cause severe ice melting and rapid ablation rates. Melting ice can remove or alter chemical signals in the ice that are valuable for climate and pollution research, including δ18O, volcanic aerosols, and major ions. These glaciers also can be difficult to date, because distinct layers are not always present. In Wyoming, even glaciers that were in close proximity had varying preservation of volcanic ash layers for example.
4. Studies of the Wind River Range glaciers in Wyoming show complex records of climate and pollution. The Little Ice Age is the most prominent climate signal, and transfer functions suggest the chance for recent rapid warming in this region. The Upper Fremont glacier is the best studied, but it took several studies to develop robust dating and understanding of the chemical signals in the ice.
references
Cecil, L. D., & Vogt, S. (1997). Identification of bomb-produced chlorine-36 in mid-latitude glacial ice of North America. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 123(1-4), 287-289.
Cecil, L. DeWayne, et al. "Isotopic composition of ice cores and meltwater from Upper Fremont Glacier and Galena Creek rock glacier, Wyoming." Geografiska Annaler: Series A, Physical Geography 80.3‐4 (1998): 287-292.
Clark, Ian D., and Peter Fritz. Environmental isotopes in hydrogeology. CRC press, 1997.
Grootes, P. M., Stuiver, M., White, J. W. C., Johnsen, S., & Jouzel, J. (1993). Comparison of oxygen isotope records from the GISP2 and GRIP Greenland ice cores. Nature, 366(6455), 552.
Johnsen, S. J., Dahl‐Jensen, D., Gundestrup, N., Steffensen, J. P., Clausen, H. B., Miller, H., ... & White, J. (2001). Oxygen isotope and palaeotemperature records from six Greenland ice‐core stations: Camp Century, Dye‐3, GRIP, GISP2, Renland and NorthGRIP. Journal of Quaternary Science: Published for the Quaternary Research Association, 16(4), 299-307.
Figure 10: Reconstructed air temperature trends during approximately 1950-1995 obtained from delta18O values a composite transfer function for the Upper Fremont Glacier, WY. (Fig. 11, Naftz et al., 2002)
Naftz, David L., James A. Rice, and James R. Ranville. "Glacial ice composition: A potential longterm record of the chemistry of atmospheric deposition, Wind River Range, Wyoming." Water resources research 27.6 (1991): 1231-1238.
Naftz, David L., Robert L. Michel, and Kirk A. Miller. "Isotopic indicators of climate in ice cores, Wind River Range, Wyoming." Climate Change in Continental Isotopic Records 78 (1993): 5566.
Naftz, David L., et al. "Little Ice Age evidence from a south-central North American ice core, USA." Arctic and Alpine Research 28.1 (1996): 35-41.
Naftz, David L., et al. "Ice core evidence of rapid air temperature increases since 1960 in alpine areas of the Wind River Range, Wyoming, United States." Journal of Geophysical Research: Atmospheres 107.D13 (2002): ACL-3.
Ruddiman, W.F., “Earth's Climate: past and future”. Macmillan. 2001
Schuster, Paul F., et al. "Chronological refinement of an ice core record at Upper Fremont Glacier in south central North America." Journal of Geophysical Research: Atmospheres 105.D4 (2000): 4657-4666.